From PROTAC to RIPTAC, it is a challenge and an opportunity
December 19, 2024
Source: drugdu
 1,371
1,371
 In 2001, Craig Crews, the former founder of Arvinas and Professor of Yale University, first proposed the concept of PROTAC, leading a new revolution in small molecule innovative drugs. In 2023, Professor Crews founded HALDA Therapeutics and unveiled their latest technology platform RIPTAC at the ASCO-GU conference. At the same time, he published the latest article on bioRxiv, describing the results of the RIPTAC proof-of-concept study. In 2024, HALDA Therapeutics announced the completion of a $126 million Series B financing, and emphasized that the funds raised will be used for clinical trials of two candidate drugs in patients with prostate cancer and breast cancer.
In 2001, Craig Crews, the former founder of Arvinas and Professor of Yale University, first proposed the concept of PROTAC, leading a new revolution in small molecule innovative drugs. In 2023, Professor Crews founded HALDA Therapeutics and unveiled their latest technology platform RIPTAC at the ASCO-GU conference. At the same time, he published the latest article on bioRxiv, describing the results of the RIPTAC proof-of-concept study. In 2024, HALDA Therapeutics announced the completion of a $126 million Series B financing, and emphasized that the funds raised will be used for clinical trials of two candidate drugs in patients with prostate cancer and breast cancer.
From PROTAC to RIPTAC, it was originally an innovative theory proposed by the same professor, but it is essentially based on two different directions. The former emphasizes the transition from "placeholder-driven" to "event-driven", while the latter returns from "event-driven" to "placeholder-driven". What is the reason? More importantly, what is the special significance of the birth of RIPTAC? Is it really a new variant of PROTAC?
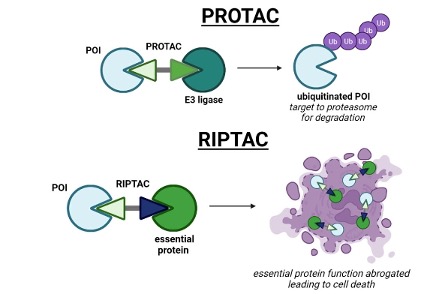
The trend from PROTAC to RIPTAC. In theory, PROTAC and RIPTAC are both members of the targeted chimera (TACs), and both are based on bifunctional small molecules. But structurally, the former is composed of target protein binding ligand + linker + E3 ubiquitin ligase binding ligand; the latter is composed of target protein binding ligand + linker + protein ligand (EP) necessary for maintaining cell survival. The difference between the two seems to be only the choice of back-end ligands, but in fact, the mechanism of action of the two is very different.
First, PROTAC enables E3 ubiquitin ligase to approach and recognize POI through the formation of a ternary complex, and then catalyzes the ubiquitination reaction. E3 ubiquitin ligase catalyzes the covalent binding of ubiquitin molecules to the lysine residues of POI to form a string of polyubiquitin chains, and finally the ubiquitin-tagged POI is recognized and degraded by the proteasome.
It was originally designed to solve the two major bottlenecks of the "occupancy drive" of traditional small molecule targeted drugs, namely: under the original regulatory mechanism, 80% of the proteins in human cells cannot find regulatable small molecules. In the process, a higher local drug concentration is required to exert the effectiveness of the drug, which increases the risk of subsequent off-target. The emergence of PROTAC has shifted the original small molecule drugs from traditional "occupancy drive" to "event drive". On the one hand, the tumor binding sites are more extensive, making more "undruggable" targets possible. On the other hand, direct degradation of the entire driver protein also makes it more difficult for tumor mutations to escape. In theory, the blocking effect of PROTAC is significantly better than the simple inhibitory effect of small molecule targeted drugs, and the drugability ceiling is also higher. This is also the fundamental reason why PROTAC was so popular in the past few years.
However, as the research on PROTAC technology deepens, its limitations as a new technology are gradually exposed. The process involves many basic mechanism problems that the industry cannot currently solve, such as: Too large molecular weight: As a dual-target drug, PROTAC has a larger molecular weight than general small molecule drugs, generally above 700 Daltons, which seriously affects the water solubility and membrane permeability of the molecule to a certain extent, making it unfavorable for oral administration.
Limited recruitment of E3 ligases: Although E3 ligases are a large protein family, the E3 ligases currently recruited by PROTAC are mostly concentrated in a few individuals such as VHL, CRBN and MDM2. The development of E3 ligases and their ligands is relatively lagging, which fundamentally limits the application of PROTAC. Difficulty in target protein degradation: When the ternary complex is formed, whether it can be effectively degraded still needs to fight against ubiquitinase and target protein resynthesis until a certain degree of balance is reached. Poor number of targeted proteins: PROTAC can target and successfully degrade less than expected proteins. In addition, there are many pharmacokinetic issues such as plasma exposure, cell entry efficiency, toxicity, blood-brain barrier, etc. that need to be considered and screened. Therefore, although the ideal of PROTAC technology is full, it is not as perfect as we imagine.
Unlike PROTAC, RIPTAC is a ternary complex in structure, but it does not kill tumors by inducing the degradation of tumor target proteins, but causes tumor cells expressing target proteins to die due to the loss of basic key functions. In a sense, RIPTAC is more like a small molecule version of ADC, and in fact, some people do associate RIPTAC with SMDC (small molecule drug conjugate) and DDC (drug-drug conjugate). Comparing the structural composition of SMDC and RIPTAC, the former uses a small molecule drug as a targeting antibody at one end, and the payload at the other end is a cytotoxic molecule; while the latter has a target protein that is highly expressed in the tumor at one end, and the EP protein ligand that determines the survival of the tumor at the other end. To some extent, RIPTAC can also do the same thing of "precision guidance", which is similar to ADC, PDC, SMDC, and even TCE bispecific antibodies.
In terms of advantages, compared with traditional small molecule drugs, RIPTAC does not form a corresponding complex due to the existence of a targeting mechanism, as there is no specific target protein in the tumor in normal cells, thereby reducing drug toxicity; and compared with conjugated drugs, RIPTAC's bifunctional small molecule characteristics also lead to a wider range of optional targets (lack of tissue and organ targeting), and a wider range of indications in theory. Overall, RIPTAC is more like a combination of conjugated drugs and small molecule drugs, with both the advantages of small molecule drugs in manufacturing and delivery, and the advantages of conjugated drugs in precise guidance and exemption of healthy cells. More importantly, RIPTAC gets rid of its dependence on cancer-driving proteins and creates new cancer drugs for patients with drug resistance.
Of course, while RIPTAC technology has solved a series of difficulties, it has also brought about more new limitations, bringing new challenges to the development of the industry, such as:
① Large molecular weight: Like PROTAC, RIPTAC is a small molecule inhibitor with a large molecular weight and changed physical and chemical properties, which makes its development more difficult, and the difficulty of oral administration still exists.
② Decreased usage rate: From a mechanism perspective, RIPTAC has abandoned the "event-driven" technical highlight that PROTAC is proud of, and has returned to the "placeholder-driven" of traditional pharmaceutical companies, which has resulted in its inability to be used repeatedly as before, and only one RIPTAC can correspond to one target protein.
③ As a new technology, whether there will be enough TP and EP for development in the future is still unknown.
From the establishment of HALDA to the exposure of RIPTAC's mechanism of action, and then to the disclosure of information on a series of drugs, RIPTAC has not been around for a long time, and it will take a long time to be fully industrialized. Compared with innovative therapies such as ADC, single/double antibodies, it is more like a newborn baby.
According to statistics, since the concept of RIPTAC's new drug was not proposed until 2023, there are not many RIPTAC new drug layouts in the global field, and the only known layout company is HALDA.
Its lead RIPTAC therapeutic drug HLD-0915 can simultaneously bind to the androgen receptor (AR) and an important cellular protein involved in transcriptional regulation. The resulting trimer complex leads to the loss of essential protein function and the selective death of prostate cancer cells. In the preclinical data of 2023, HLD-0915 has a selective killing effect on prostate cancer cells in vitro and anti-tumor activity in rodent models of prostate cancer in vivo.
① It can selectively induce apoptosis of ARhi prostate cancer cells.
② It has preclinical activity in Enzalutamide-resistant models.
③ By pre-administering AR LBD inhibitors, AR-dependent EP inhibition is verified (pre-administering Enzalutamide competes with RIPTAC for AR binding, thereby blocking trimer complex formation and EP inhibition).
④ RIPTAC downregulates genes involved in homologous recombination repair, induces BRCAness, and will explore combined treatment with PARP inhibitors.
The latest news shows that HLD-0915 is expected to start a Phase 1 clinical trial in the first half of 2025 for the treatment of patients with metastatic, castration-resistant cancer (mCRPC). At the same time, HALDA completed a new round of financing in August 2024, and the funds will also be used to support the clinical development of its second RIPTAC for the treatment of metastatic cancer and further build a team. It can be expected that with the clinical progress of more new drugs such as HLD-0915 in the future, the drugability of RIPTAC technology will be further verified. Perhaps in 2025, more biotech companies and investment companies will join the game, and there is even hope to see BD transactions facilitated by MNC.
At present, China's attitude towards RIPTAC drugs is relatively cautious. Although leading biotech companies such as BeiGene have noticed this technology therapy, since the technology is still in the technical verification stage, the risk of entry is too high. Therefore, whether it is enterprises, investment institutions, or even universities (no Chinese literature has yet involved) The action in this field is limited to discussion. Instead, the PROTAC and molecular glue fields before this are more popular.
Taking PROTAC as an example, Chinese biotech companies have a huge number of related pipelines, and some innovative therapies have even entered the world's leading level, such as Haichuang Pharmaceuticals, Kintor Pharmaceuticals, BeiGene, Ruiyue Bio, Shennuo Bio, etc., and Chinese pharmaceutical companies are also paying attention, such as Haisike, Kintor Pharmaceuticals, Haichuang Pharmaceuticals, Innovent Biologics, Hengrui Medicine, etc.
At this point, considering the homology of PROTAC and RIPTAC, the most likely scenario for RIPTAC in the future is to develop in coordination with PROTAC, which means that the above-mentioned PROTAC companies are likely to become RIPTAC's biggest potential stocks in the future, realizing a seamless connection from PROTAC to RIPTAC. From small molecule drugs to PROTAC, and then from PROTAC to RIPTAC, it is the ultimate manifestation of the continuous change in demand in the biopharmaceutical industry.
The emergence of PROTAC makes undruggable targets possible, and also gives small molecule drugs the hope of re-emerging in the era of the prevalence of large molecule drugs. RIPTAC, on the other hand, introduces a completely new mechanism based on PROTAC, making it possible to get rid of the dependence on cancer-driving proteins and create new cancer drugs for patients with drug resistance.
However, as far as the current RIPTAC technology is concerned, its development is still in a very early stage, and its research is mainly focused on the exploration of basic mechanisms of action. However, as the current trend of precision treatment is increasingly recognized, the shift from small molecule targeted drugs to pioneering drug discovery strategies such as RIPTAC has also become a new possibility. More importantly, for most Biotechs struggling with "involution" today, the development of RIPTAC as a potential viable treatment for a series of diseases is an exciting prospect. The current blank market may also explode with market potential no less than that of bispecific antibodies, ADCs, etc. in the future with the joint efforts of capital, researchers and enterprises. Chinese companies may seize the opportunity.
https://news.yaozh.com/archive/44688.html
By editorRead more on
- InnoCare Receives First-in-China Clinical Approval for VAV1 Molecular Glue Degrader February 10, 2026
- End of Overseas Partnership for Compound Danshen Dripping Pills Deals, Another Blow to Tasly’s “American Dream” February 10, 2026
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



