Domestic dual antibody makes another breakthrough
November 9, 2024
Source: drugdu
 242
242
Introduction: Has the best potential in its class
Recently, Weili Zhibo announced that its independently developed Class 1 new drug LBL-034, which has global intellectual property rights, has been granted orphan drug status by the US Food and Drug Administration (FDA) for the treatment of multiple myeloma (MM).
LBL-034 is a new generation of humanized bispecific T cell linker antibody targeting GPRC5D and CD3, developed using LeadsBody TM, a CD3 bispecific technology platform independently developed by Weili Libo with intellectual property rights. It is the third CD3 T cell linker targeting GPRC5D to enter the clinical stage worldwide.
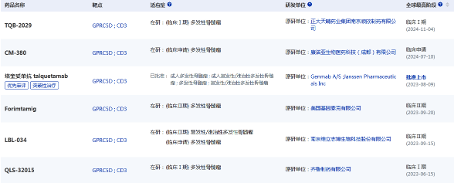 Figure 1: GPRC5D/CD3 dual antibody under global research
Figure 1: GPRC5D/CD3 dual antibody under global research
Image source: Pharmaceutical Intelligence Data
Multiple myeloma (MM) is a malignant plasma cell disease caused by abnormal proliferation of clonal plasma cells, accounting for approximately 10% of hematological malignancies and 1% of all tumor diseases. MM is still an incurable malignant tumor. With multi line medication, the recurrence interval will become shorter and eventually evolve into RRMM, which seriously threatens the patient's life and health. Developing new and more effective treatment plans is urgent.
According to a press release from Weili Libo, LBL-034 selectively binds to T cells only in the presence of GPRC5D+cells, thereby conditionally activating T cells in the tumor microenvironment where GPRC5D is expressed. In cells with high, medium, and low GPRC5D expression, LBL-034 consistently exhibits excellent targeted tumor cell killing activity and strong dose-dependent anti-tumor activity, demonstrating the potential to become the best choice for similar anti-tumor drugs.
LBL-034 was approved for clinical trials in China and the United States in July 2023, and an open label, multicenter, dose escalation/expansion phase I/II study was initiated in China in November 2023 for patients with relapsed/refractory multiple myeloma (RRMM). Weili Libo will present the preliminary clinical data of this study at the 66th Annual Meeting of the American Society of Hematology (ASH) in 2024.
Read more on
- 20-Valent Pneumococcal Vaccine Approved for Clinical Trials January 20, 2026
- ADC205 Tablets Received Approval for Drug Clinical Trial January 20, 2026
- Sifang Optoelectronics makes strategic investment in Changhe Biotechnology January 20, 2026
- Mingde Bio plans to acquire a 51% stake in Hunan Lanyi through capital increase and acquisition January 20, 2026
- accelerating the transition of CLL treatment into a “chemotherapy-free era”. January 20, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



