Two innovative medical devices have been approved for market launch
November 6, 2024
Source: drugdu
 229
229
 Recently, the National Medical Products Administration announced the approval of the registration applications for two innovative products, Yabonixi Medical Technology (Suzhou) Co., Ltd.'s "knee joint prosthesis system" and Shanghai Xinwei Medical Technology Co., Ltd.'s "intracranial aneurysm embolization assisted stent".
Recently, the National Medical Products Administration announced the approval of the registration applications for two innovative products, Yabonixi Medical Technology (Suzhou) Co., Ltd.'s "knee joint prosthesis system" and Shanghai Xinwei Medical Technology Co., Ltd.'s "intracranial aneurysm embolization assisted stent".
Yabonixi Medical offers freedom of movement in all aspects
Yabonixi Medical is a wholly-owned subsidiary of Jiaoying Medical Equipment (Shanghai) Co., Ltd., dedicated to the research, development, production, and sales of high-end orthopedic medical equipment, minimally invasive surgical medical equipment, and more. Yabonixi Medical was founded by Dr. Yao Jianqing, a leading figure in the international orthopedic field.
It is reported that Yao Jianqing is an experienced veteran in the field of medical devices, with 36 years of rich experience in medical device and tissue engineering research and industrialization. He has served as the Global R&D Senior Director and Asia Pacific R&D Senior Director of ZimmerBiomet, a joint company. Yao Jianqing has participated in/led the development of a series of products, including high cross-linked wear-resistant polyethylene materials, hinge and rotating platform artificial knee joints, rotator cuff ligament repair biological patches, cartilage regeneration products, minimally invasive surgical endoscopic staplers, etc., which have successfully entered the international and domestic markets.
Yabonixi Medical relies on the technological accumulation of the company's R&D team in the fields of orthopedics and minimally invasive surgical medical devices, adheres to the product development route of "new technology new device", deeply cultivates orthopedics and minimally invasive surgery, and aims to meet the historical opportunity of import substitution in the rapidly growing domestic market with high-quality and cost-effective products, as well as undertake the historical mission of Chinese made high-end medical devices entering the European, American, Japanese, and Australian markets. At present, Yabonixi Medical has developed multiple patented technologies and industrialized products such as artificial hip joints, artificial knee joints, and endoscopic staplers.
The knee prosthesis system approved for market this time consists of femoral components, tibial components, and patellar components, suitable for first-time knee replacement surgery in patients with mature bone development. Among them, the interface between the femoral condyle and tibial tray and the bone is composite with a porous structure of bone trabeculae made by additive manufacturing technology, which has high friction coefficient and good bone bonding performance.
Moreover, the knee prosthesis system achieves multi-dimensional freedom of movement. The unique design of the femur and ankle is saddle shaped, which increases the stability of the knee joint; The cushion cleverly cooperates with the femur ankle to ensure smooth flexion, extension, sliding, and rotation; The tibial plateau adopts a T-shaped structure to provide reliable fixation support; The lower surface of the platform support adopts a porous structure to promote bone growth and make the prosthesis more stable and durable. With the help of this product, joint movements can be more natural and smooth, providing support for patients to restore their health and mobility.
Xinwei Medical, innovative product landing
Founded in 2016, Xinwei Medical is committed to improving the accessibility of medical innovation technology and products, reducing the mortality rate of major diseases, improving patient prognosis, and safeguarding life and health. At present, Xinwei Medical has pioneered the development of a one-stop solution for stroke treatment and prevention in the field of neural intervention. Its product pipeline covers nerve intervention treatment equipment such as thrombectomy, aneurysm and stenosis, intervention pathway equipment, and stroke prevention equipment.
Xinwei Medical has nearly 30 domestic medical device product registration certificates, more than 200 authorized patents, and 2 products have obtained the qualification of "priority evaluation of innovative devices". The intracranial aneurysm embolization auxiliary stent is the second product of Xinwei Medical to enter the special examination procedure for innovative medical devices after the rapamycin coated intracranial drug balloon catheter.
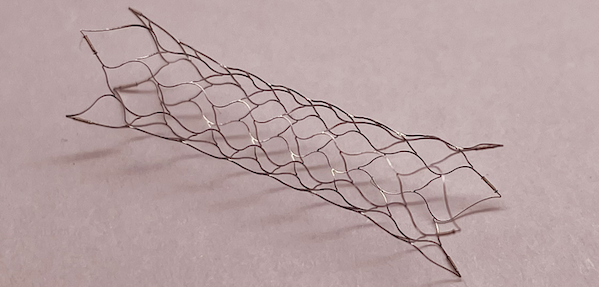
The intracranial aneurysm embolization assisted stent approved for market this time consists of three parts: stent, delivery guide wire, and introduction sheath. The stent part is made of nickel titanium alloy tube material laser engraved, and there are platinum iridium imaging points at both ends of the stent and in the middle of some models, which facilitate accurate clinical judgment of stent opening and adhesion. Under clinical standard interventional surgical conditions, the location of intracranial aneurysms is determined based on angiography, which is used for intravascular embolization and blood flow reconstruction in patients with intracranial aneurysms.
The intracranial aneurysm embolization assisted stent has innovative design, with a "mid section visualization" intracranial self expanding laser engraving stent. The imaging points are scattered in the middle of the stent, which can effectively clarify the relative position relationship between the stent and the aneurysm neck, ensuring that the stent provides the strongest support for the spring coil; After the bracket is released, its adhesion effect can be observed through the developing point in the middle of the bracket.
Moreover, the intracranial aneurysm embolization assisted stent has a wider range of product specifications. The newly added 60mm length stent and 3.0mm diameter stent are suitable for blood vessel diameters ranging from 1.5mm to 3.0mm, providing clinical doctors with more choices.
Source: https://news.yaozh.com/archive/44483.html
By editorRead more on
- 20-Valent Pneumococcal Vaccine Approved for Clinical Trials January 20, 2026
- ADC205 Tablets Received Approval for Drug Clinical Trial January 20, 2026
- Sifang Optoelectronics makes strategic investment in Changhe Biotechnology January 20, 2026
- Mingde Bio plans to acquire a 51% stake in Hunan Lanyi through capital increase and acquisition January 20, 2026
- accelerating the transition of CLL treatment into a “chemotherapy-free era”. January 20, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



