GCGR agonist rejected twice
October 15, 2024
Source: drugdu
 228
228
Original Time Biopharmaceutical Editor October 14, 2024 09:20 Shanghai
The weight loss drug concept stock Zealand has suffered another setback, as its new drug Dasiglucagon, which was launched to treat congenital hyperinsulinemia (CHI) in children, has once again been rejected by the FDA.
Last December, Dasiglucagon was rejected by the FDA for the first time due to the discovery of defects in a third-party contract manufacturing factory.
Zealand pointed out that the main reason for Dasiglucagon's second rejection was that the FDA failed to complete the re inspection of third-party production factories, resulting in the approval process being put on hold. The FDA did not raise any concerns about the effectiveness or safety of Dasiglucagon.
Dasiglucagon is a glucagon receptor (GCGR) agonist that acts to release stored glycogen from the liver into the bloodstream.
Hypoglycemia is one of the most common acute complications of diabetes, and patients who have the ability to continuously monitor blood glucose cannot completely prevent serious hypoglycemic events.
Compared with patients without severe hypoglycemia, type 2 diabetes patients with severe hypoglycemia have an increased risk of heart failure, atherosclerotic cardiovascular disease, all-cause mortality and transient ischemic attack. In addition, recurrent hypoglycemia may exacerbate patients' cerebral ischemic damage and affect their immune response.
In 2021, Dasiglucagon was approved by the FDA to be marketed for the treatment of severe hypoglycemia in diabetes patients aged 6 years and above. In June 2022, Zealand and Novo Nordisk entered into a global licensing and development agreement for Dasigligagon, with a total transaction amount of $45.75 million. Dasigligagon entrusted the global commercialization of the drug to the latter.
Zealand was founded in 1998, and the company is only a few miles away from another company, Novo Nordisk. Several management members, including the CEO of Zealand, have also worked at Novo Nordisk.
In 2010 and 2017, Zealand was listed on the Copenhagen Stock Exchange in Denmark and on the NASDAQ Stock Exchange in the United States.
Initially, Zealand also focused on lowering blood sugar. Its first marketed product, Lysiral, was a GLP-1 receptor agonist, but it was licensed to Sanofi in 2003.
In recent years, due to the popularity of weight loss drugs and the poor sales performance of Dasiglucagon, Zealand has also changed its research and development direction, focusing on the development of weight loss drugs.
It can be said that this strategic shift is crucial for the revival of Zealand. Driven by the positive clinical data of weight loss drugs, the company's stock price has risen by 158% in the past year.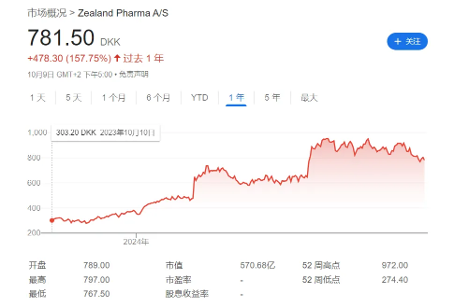 Zealand has laid out multiple pipelines around weight loss, among which Survodutide, the fastest, has advanced to phase III clinical stage. Survodutide is a GCGR/GLP-1R dual target agonist developed in collaboration with Boehringer Ingelheim, with indications including weight loss and MASH.
Zealand has laid out multiple pipelines around weight loss, among which Survodutide, the fastest, has advanced to phase III clinical stage. Survodutide is a GCGR/GLP-1R dual target agonist developed in collaboration with Boehringer Ingelheim, with indications including weight loss and MASH.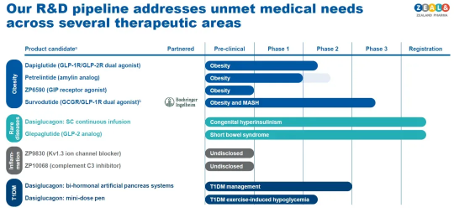 Zealand has also developed the world's first GLP-1/GLP-2 dual agonist Dapiglutide, which is currently in phase II clinical trials.
Zealand has also developed the world's first GLP-1/GLP-2 dual agonist Dapiglutide, which is currently in phase II clinical trials.
Last month, Zealand released phase II clinical data on the use of dapiglutide for weight loss. At week 13, the Dapiglutide group achieved a maximum weight loss of 6.2%, while the placebo group gained 2.1%.
There are also two weight loss pipelines - Petrelinide, an amygdalin analogue, and ZP6590, a GIP receptor agonist.
Because Dasiglucagon did not sell well and Zealand was also short of money, the company signed a 90 million euro loan financing agreement with a European investment bank last year.
This year, the company's financial situation has significantly improved, and its positive performance in the stock market has led the company to raise 7 billion Danish kroner (approximately 1 billion US dollars) through an additional issuance in June of this year, relieving the urgent need for funds.
At the end of last year, Zealand also submitted a marketing application for another new drug to the FDA - glepaglutide, a long-acting glucagon like peptide-2 (GLP-2) analog used to treat adult patients with short bowel syndrome who rely on parenteral nutrition support.
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



