The role of brainstem medullary circuit in rapid eye movement sleep and Parkinson’s disease
October 2, 2024
Source: drugdu
 296
296
Mammalian sleep includes rapid eye movement sleep (REMS) and non rapid eye movement sleep (NREM). REMS is also known as paradoxical sleep. The characteristic of NREM is the high amplitude slow wave oscillation of the electroencephalogram, which reflects the synchronous transition of cortical neurons between depolarization rising state and hyperpolarization falling state. In contrast, REM is characterized by an increase in the average firing rate of cortical neurons, rapid oscillations and theta waves in the electroencephalogram, and loss of skeletal muscle activity (muscle tone imbalance). The brainstem's brainstem is a key structure regulating REMS.

Research on cats has shown that the perifocal coeruleus (LC) - α is a crucial region within the pontine tegmentum for inducing REM. Subsequent analysis showed that the subthalatoral nucleus (SLD) of rodents is homologous to LC - α in cats [6,7]. In SLD, inhibiting glutamatergic neurotransmission can reduce REMS levels and alleviate muscle tone disorders. The glutamatergic neurons in this region are highly heterogeneous, while the neurons that promote non rapid eye movement or wakefulness exhibit a mixed state. Although the neuronal entity responsible for REM in SLD is still unclear, recent studies have revealed the role of neurons in promoting REM in other regions, including melanocyte concentration hormone producing neurons in the lateral hypothalamus, GABAergic neurons in the ventral medulla, and dopaminergic neurons in the ventral tegmental area. And these findings suggest that REM is controlled by a dispersed network of neurons throughout the brain, rather than a specific central loop. Of particular note is that the ablation/chronic inactivation of these neurons has limited impact on REMS levels. On the contrary, lesions in the brainstem region can lead to almost complete loss of REMS [3,5], indicating that SLD does play a central role. Therefore, it is crucial to identify the neuronal subtypes that cause REMS in SLD and reveal their neural circuits.
In recent years, the defects of REMS, especially REMS behavior disorder (RBD), have attracted widespread attention due to their correlation with synucleinopathy. RBD is a sleep disorder characterized by bodily movements in dreams during REM due to muscle tone disorders. Queue studies have shown that RBD is a precursor marker for synucleinopathies, including Parkinson's disease (PD). In addition to RBD, PD patients often experience excessive sleepiness and insomnia, and the characteristic of sleep deprivation in PD patients is the progressive decline of REM and NREM. Considering that the late stage of synucleinopathy is often accompanied by cognitive impairment, and memory consolidation requires REM, it is crucial to elucidate the reasons for sleep deprivation in synucleinopathy. Although neuronal degeneration and Lewy bodies (aggregates of alpha synuclein) were observed in the brainstem of PD patients, the lack of neuronal molecular markers that control sleep has limited their precise functional studies in sleep related symptoms.
On September 19, 2024, Yu Hayashi's research group from the University of Tsukuba in Japan published an article titled "A pontine modular loop critical for REM sleep and its deficiency in Parkinson's disease" in Cell, discovering that a neural circuit originating from the brainstem and medulla can induce REMS.
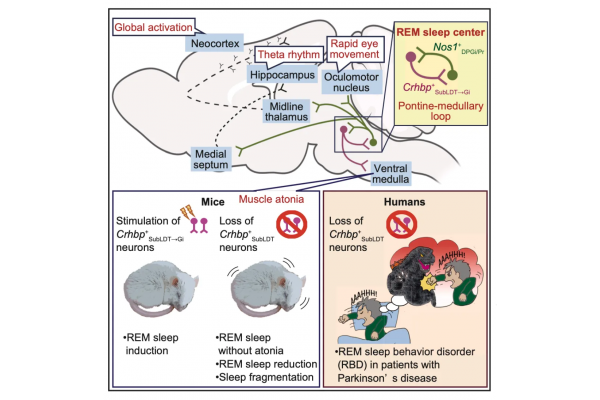
Due to the heterogeneity of brainstem neurons, identifying the characteristics of rapid eye movement (REM) sleep circuits and their relationship with diseases has always been challenging. In this study, based on transcriptomic analysis of the brainstem canopy, the authors identified a specific neuronal subtype that regulates muscle tone during REMS/NREMS and REMS. The author found in mice that the expression of Crhbp (corticotropin releasing hormone binding protein) in the brainstem olfactory tegmental and projecting it to neurons in the medulla (i.e. Crhbp+neurons) can promote REM. In the medullary region receiving projections from Crhbp+neurons, neurons expressing nitric oxide synthase 1 (Nos1+neurons) project to the brainstem olfactory cortex and promote REM, indicating a positive interaction circuit between the brainstem and medulla, and that this neural circuit can cover the eye movement sleep circuit.
Nos1+neurons also project to areas that control extensive forebrain activity. Removing Crhbp+neurons can reduce sleep and impair REMS. In Parkinson's disease patients with REMS behavioral disorders, the immune response level of Crhbp+neurons is significantly reduced and contains pathological alpha synuclein, providing new insights into the mechanism of sleep defects in this disease.
In summary, the author found that the neural circuit derived from the brainstem "Crhbp+neurons and medulla Nos1+neurons" can induce REMS, even in awake mice. In addition, patients with Parkinson's disease have a deficiency of Crhbp+neurons. This work establishes a neural genetic pathway that promotes REMS, providing a theoretical basis for further studying its distribution, upstream and downstream circuits, and its correlation with human sleep disorders.
By editor
Read more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



