EGFR mutated NSCLC has an ORR of 80%! Zhikang Hongyi CDH3 ADC announces latest clinical results
September 25, 2024
Source: drugdu
 244
244
Insight Database On September 14th, Zhikang Hongyi announced the latest clinical data (NCT05957471) on the safety and efficacy of its globally exclusive antibody conjugated drug BC3195 (CDH3 ADC) in phase I clinical trials for advanced solid tumors at the 2024 ESMO Annual Meeting. The ORR for patients carrying EGFR mutations reaches 80%. Image source: ESMO official website
Image source: ESMO official website
BC3195 uses antibodies with high affinity for CDH3 protein and exhibits good endocytic activity, as well as clinically validated linkers and effective payload vc MMA with bystander effect.
The data released this time shows that BC3195 has controllable security and good PK characteristics. BC3195 has shown significant anti-tumor activity in NSCLC patients, with an overall response rate (ORR) of 36.4%, especially for patients carrying EGFR mutations with an ORR of 80%.
The data deadline is August 10, 2024. This phase I clinical study enrolled a total of 34 patients, all of whom were late stage patients undergoing multi line treatment. Among them, 3 patients were enrolled in each of the four dose groups of 0.3, 0.6, 1.2, and 1.8 mg/kg Q3W, 21 patients were enrolled in the 2.4 mg/kg Q3W dose group, and 1 patient was enrolled in the 1.2 mg/kg QW dose group.
Among the 30 patients with assessable tumor response, no complete response (CR) or partial response (PR) was reported in the dose group of 1.8 mg/kg Q3W and below
In the 2.4 mg/kg Q3W dose group, the best response of 5 patients was partial remission, and all were confirmed (cPR), including 4 patients with non-small cell lung cancer and 1 patient with breast cancer.
Among 11 non-small cell lung cancer (NSCLC) patients treated with 2.4 mg/kg, 10 patients experienced tumor volume reduction, of which 4 patients were confirmed to have partial response (cPR), and 6 patients had the best response of disease stability (SD), with ORR and DCR of 36.4% and 90.9%, respectively; Among the 5 NSCLC patients carrying EGFR mutations, 4 cases were confirmed to have PR (cPR), with an ORR of 80%. One patient who obtained cPR had undergone 5-line treatment, and PR remission had lasted for 20 weeks.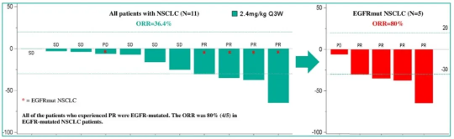 BC3195 at a dose level of 2.4 mg/kg Q3W
BC3195 at a dose level of 2.4 mg/kg Q3W
The best therapeutic effect for NSCLC
In terms of safety, only one dose limiting toxicity (DLT) event, grade 3 pharyngitis, occurred in the 2.4 mg/kg Q3W dose group among the 34 patients. The main adverse events (AEs) are rash, stomatitis, and abnormal liver function tests. Most of the adverse events of rash and stomatitis occur in the first dosing cycle, and their safety is controllable
Fourteen patients (41.2%) experienced treatment-related adverse events (TRAEs) of grade ≥ 3, with more than two patients developing stomatitis (23.5%), decreased neutrophil count (8.8%), and rash (8.8%).
BC3195 is currently conducting Phase I dose optimization and dose expansion studies simultaneously in China and the United States.
By editorRead more on
- Gan & Lee Pharmaceuticals’ new PROTAC drug GLR2037 tablets have been approved for clinical trials to enter the field of prostate cancer treatment March 3, 2026
- AideaPharmaceuticals plans to raise no more than 1.277 billion yuan through a private placement to focus on the global clinical development of innovative HIV drugs March 3, 2026
- Giant Exits! Its Star Business Acquired March 3, 2026
- Focusing on cardiovascular and cerebrovascular diseases! OpenMediLead Medical Intelligence Dual Engines Launch Internal Testing, Connecting Drug Development and Clinical Diagnosis in a Closed Loop March 3, 2026
- Innovent Biologics Announces Approval of New Indication for BTK Inhibitor “Pitubrutinib” in China March 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



