Two innovative medical devices from Medtronic have been approved for market in China
September 13, 2024
Source: drugdu
 677
677
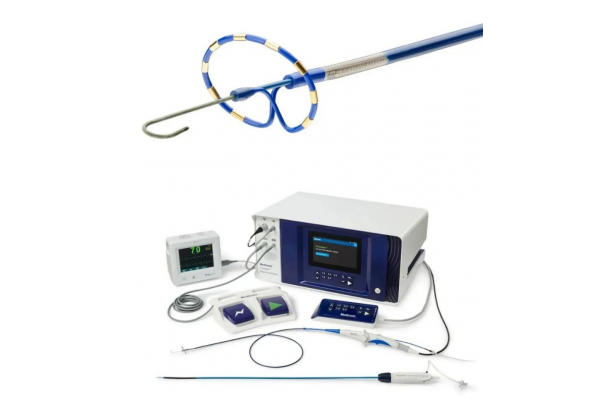
Recently, the National Medical Products Administration announced the approval of Medtronic's registration applications for two innovative products, the "Heart Pulse Electric Field Ablation Generator" and the "Disposable Heart Pulse Electric Field Ablation Catheter".
Unique advantages, suitable for two indications
It is reported that the two innovative products approved this time are Medtronic's PFA product - PulseSelect, which is the first PFA product approved by NMPA that can be used for both paroxysmal and persistent atrial fibrillation.
PulseSelect is a new technique for treating atrial fibrillation (AF) using pulsed electric fields, which can provide bipolar and biphasic pulsed electric fields through a circular multi electrode array catheter. This technology consists of five parts: a controllable multi electrode annular ablation catheter, a PFA host ECG-Gated、 Tableside control、10Fr bidirectional sheath.
It is worth mentioning that PulseSelect's controllable multi electrode annular ablation catheter features a proprietary biphasic waveform, unique built-in guide wire, and a 20 ° forward tilt design, which supports surgical operability, reliability, and safety. According to Medtronic, the average total energy delivery time for a single pulmonary vein isolation is 30 seconds to isolate all veins. The isolation time and ability have been confirmed in large-scale clinical trials of PULSED AF.
And PulseSelect uses pulsed electric fields to ablate or create damaged and scar tissue, interrupting irregular electrical pathways in the heart and triggering factors for atrial fibrillation. However, unlike traditional ablation methods such as radiofrequency ablation or cryoablation, PulseSelect uses non thermal ablation methods that prioritize targeting cardiac tissue and selectively target myocardial cells to avoid unnecessary damage to surrounding tissues, thereby avoiding complications caused by traditional ablation methods.
PulseSelect also has its outstanding advantages among the four PFA products currently approved by NMPA. The four PFA products currently approved are: Jinjiang Electronics' PulsedFA, Deno Electrophysiology's CardiPulse, Boston Scientific's FARAPULSE, and Medtronic's PulseSelect.
Each of the four products has its own differences. In terms of category, Jinjiang Electronics and Medtronic's products are ring-shaped ablation catheters, while Deno Electrophysiology and Boston Scientific's products are petal shaped ablation catheters. Each of the four products has its own advantages, with Medtronic's product having the most extensive indications. The other three products have indications for paroxysmal atrial fibrillation, while persistent atrial fibrillation is currently under clinical research.
In addition, Medtronic's products have shown excellent clinical efficacy. According to Medtronic's PulseSelect IDE (PULSED AF) study data released last year, PulseSelect exceeded expected safety and efficacy targets, with a safety event rate of 0.7% and a clinical success rate of 80% for patients with paroxysmal and persistent AF.
Intensify the layout, and the development momentum of multiple businesses is rapid
In March 2024, Geoff Martha, Global Chairman and CEO of Medtronic, stated that Medtronic's next step is to increase investment in China and build new innovation centers.
As one of the first multinational medical technology companies to enter China during the early stages of reform and opening up, Medtronic has been developing in China for 35 years. As of March 2024, Medtronic has introduced over 700 innovative products, with one research and development center, two innovation centers, five production bases, and six factories. It has built a full value chain layout from research and development, production, sales, training, after-sales, and investment funds, and China has become Medtronic's second largest single market globally.
According to public information, so far, Medtronic China R&D Center has developed a total of 65 products, of which more than 50 have been successfully launched and 23 have achieved global sales.
In addition, Medtronic's performance this year has also been impressive. On August 20th, Medtronic released its first quarter financial report for the fiscal year 2025, which showed revenue of $7.9 billion and adjusted revenue of $8 billion, representing a growth of 2.8% and an organic growth of 5.3%.
Looking at it by section:
The cardiovascular product portfolio has shown stable performance, with revenue of 3.007 billion US dollars, a year-on-year increase of 5.5%, and organic growth of 6.9%. The Heart Failure Division (CRHF) and Structural Heart and Aortic (SHA) businesses achieved high single digit growth, while the Peripheral Vascular (CPV) business achieved medium single digit growth;
The neuroscience product portfolio is also continuously growing, with revenue of $2.317 billion, an increase of 4.4%, organic growth of 5.3%, and high single digit growth in pain therapy business;
The combination of internal and external scientific products slightly declined, with revenue of 1.996 billion US dollars, a year-on-year decrease of 0.4%, and organic growth of 1.0%, with net profit growth in the low single digits;
The diabetes product portfolio grew significantly, with revenue of $647 million, up 11.8% year on year, and organic revenue increased 12.6%.
Based on this, Medtronic has raised its revenue growth and earnings per share guidance for fiscal year 2025, with organic revenue growth guidance for fiscal year 2025 raised to 4.5% -5%, compared to 4% -5% previously.
Medtronic has added two new players to its electrophysiology market in China. Pulse field ablation, as the most promising next-generation atrial fibrillation ablation technology in the electrophysiology field, has attracted multiple medical device giants such as Johnson&Johnson, Medtronic, Abbott, and others. There are also strong enemies such as Jinjiang Electronics and Deno Electrophysiology in China. Can PulseSelect lead Medtronic to "break through the encirclement"?
Source: https://news.yaozh.com/archive/44208.html
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



