Novo Nordisk applies for listing of growth hormone in China once a week
September 7, 2024
Source: drugdu
 265
265
Original Medicine Guanlan Medicine Guanlan September 5, 2024 08:35 Shanghai
Reported by WuXi AppTec Content Team
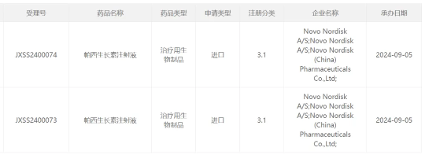
Today (September 5th), the China National Medical Products Administration's Center for Drug Evaluation (CDE) announced on its official website that Novo Nordisk's application for the new drug market launch of Paxin Injection has been accepted. According to public information, this should be the weekly long-acting growth hormone Sogroya developed by Novo Nordisk ® (Somapacitan). The Phase 3 study of this product in China for the treatment of children with slow growth caused by insufficient secretion of growth hormone has been completed.
Sogroya ® (somapactan) injection is a human growth hormone analog containing growth hormone similar to that produced by the human body, which can be used to treat adult growth hormone deficiency (AGHD). Sogroya ® has been approved in the United States, European Union, Japan, Australia, and Saudi Arabia for subcutaneous injection once a week as a replacement therapy for endogenous growth hormone in adult growth hormone deficiency (AGHD) patients. This product can also be used to treat children aged 2.5 and above who do not have sufficient growth hormone.
In 2022, Novo Nordisk announced the weekly injection of Sogroya ® The research results of somapactan have helped children achieve their annualized growth rate (AHV) growth target.
In 2020, somapacitan injection was approved for clinical use in China, intended for the treatment of pediatric patients with slow growth caused by insufficient secretion of endogenous growth hormone. Childhood growth hormone deficiency is a rare disease characterized by insufficient circulating growth hormone in the body. Growth hormone plays an important role in growth, muscle and bone strength, and helps control glucose and lipid levels in the body. Lack of growth hormone can lead to slow growth, short stature, and other health problems.
According to the official website of the Chinese Drug Clinical Trial and Information Disclosure Platform, Novo Nordisk has conducted at least two studies on somapactan injection in China, one of which is a phase 3 clinical study on the treatment of children with growth retardation caused by insufficient secretion of growth hormone. This is a Phase 3 clinical study in China, which enrolled 110 participants. Another international multicenter (including China) Phase 3 study focuses on children with short stature caused by being born as a small for gestational age (SGA) or idiopathic short stature (ISS) or Turner syndrome or Noonan syndrome, as well as evaluating the long-term safety of somapacitan.
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



