How to select T cell targeted antibodies in the strategy of antibody conjugated LNP (Ab LNP) for mRNA CAR-T therapy
September 4, 2024
Source: drugdu
 350
350
Biological Products Circle September 3, 2024 09:20 Hubei
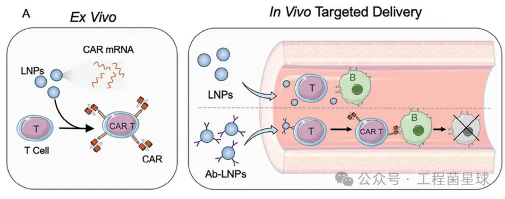
The following article is from the Engineering Bacteria Planet, written by the author Yaojun, who has doubled in number Currently, the FDA has approved multiple chimeric antigen receptor (CAR) - T-cell therapies for cancer immunotherapy. However, the production technology based on viral vectors and in vitro cell culture has led to high production costs and potential long-term side effects. With the development of mRNA and lipid nanoparticle (LNP) technology, the idea of in vivo delivery of CARs based on LNP, a non viral vector, is expected to be realized. The primary challenge faced by mRNA LNP mediated CAR-T therapy due to its liver tropism is how to achieve mRNA T cell targeting
Currently, the FDA has approved multiple chimeric antigen receptor (CAR) - T-cell therapies for cancer immunotherapy. However, the production technology based on viral vectors and in vitro cell culture has led to high production costs and potential long-term side effects. With the development of mRNA and lipid nanoparticle (LNP) technology, the idea of in vivo delivery of CARs based on LNP, a non viral vector, is expected to be realized. The primary challenge faced by mRNA LNP mediated CAR-T therapy due to its liver tropism is how to achieve mRNA T cell targeting
The use of antibody modified LNP (also known as antibody conjugated LNP, Ab LNP) is an effective solution. So, what are the markers on the surface of T cells and how do we choose antibodies targeted by T cells?
According to bacterial research, Drew Weissman (Nobel laureate) and Michael J. Mitchell from the University of Pennsylvania have published multiple conceptual validation results of Ab LNP targeting T cells for in situ CAR-T therapy. In their published literature, the bacteria found "antibody types targeting T cells", including CD4 antibodies, CD3 antibodies, CD5 antibodies, and CD7 antibodies.
01
CD4 antibody LNP delivery reporter gene mRNA
Title: Highly efficient CD4+T cell targeting and genetic recombination using engineered CD4+cell growing mRNA LNPs
Journal: Molecular Therapy [Impact Factor 12.1]
Link: pubmed.ncbi.nlm.nih.gov/34091054/
This article was published in collaboration between Weissman from Peking University and Acuitas Therapeutics, a leading mRNA company. The study reported that coupling CD4 antibodies with LNP can achieve specific targeting and mRNA targeted delivery to CD4+cells (including T cells). The mRNA used in the article encodes luciferase (reporter gene) and Cre recombinase.
After systemic injection into mice, CD4 targeted radioactive mRNA LNP accumulated in the spleen. Compared with non targeted mRNA LNP, the signal intensity of reporter gene mRNA in T cells isolated from the spleen is about 30 times higher. After intravenous injection of CD4 Ab LNPs loaded with mRNA encoding Cre recombinase into mice, specific dose-dependent loxP mediated genetic recombination was achieved, with approximately 60% and 40% of CD4+T cells expressing reporter genes in the spleen and lymph nodes, respectively. T cell phenotype analysis showed that T cell subsets were uniformly transfected.
02
CD5 antibody coupled LNP delivery of CAR mRNA
Title: CAR T cells produced in vivo to treat cardiovascular injury
Journal: Science [Impact Factor 44.7]
Link: pubmed.ncbi.nlm.nih.gov/34990237/
This article was jointly published by multiple research groups including Weissman from Peking University. The study reported that LNP modified with CD5 antibody successfully delivered CAR mRNA to mouse T cells and produced effective CAR-T cells. The CAR mRNA sequence contains the scFv fragment of a mouse fibroblast activation protein (FAP) specific monoclonal antibody.
Researchers injected LNP mRNA targeting CD5 into a mouse model of heart failure and evaluated the efficacy of in vivo CAR-T cell therapy. The experiment observed that Ab LNP successfully delivered CAR mRNA efficiently to T lymphocytes, producing transient and effective CAR-T cells in vivo.
The breakthrough of this study lies in the construction of an in vivo CAR-T platform, which can induce the in vivo production of CAR-T cells without the need to isolate cells from the patient's body, and can be applied to different diseases.
03
CD3/CD5/CD7 antibody coupled LNP delivery of CAR mRNA
Title: In Vivo mRNA CAR T Cell Engineering via Targeted Ionizing Lipid Nanoparticles with Extrahepatic Tropism
Journal: Small [Impact Factor 13]
Link: pubmed.ncbi.nlm.nih.gov/38072809/
This study was co published by Michael J Mitchell and Weissman from the University of Pennsylvania, with Michael J Mitchell as the corresponding author.
The author mentioned in the research background that T cell markers include CD3, CD8, CD4, CD7, CD5, Nrp1, and β 7. Among them, CD4 and CD8 are markers of T cell subsets, which may be unfavorable for applications such as CAR-T cell immunotherapy. CD3, CD5, and CD7 are pan T cell markers that are more suitable for CAR-T therapy. Therefore, the researchers used three types of antibodies to modify LNP for delivering CD19 targeted CAR mRNA.
The results of in vivo experiments in mice showed that delivering CAR mRNA to two Ab LNP platforms targeting CD3 and CD7 resulted in significant CAR positive cells, accompanied by effective B cell depletion. Therefore, the author believes that these two Ab LNPs are a means of producing functional CAR T cells in vivo, which can be developed and applied to CAR T cell engineering and other T cell engineering platforms.
04
summary
The CAR-T cell therapy currently approved for market is an in vitro T cell programming that has achieved significant success in treating hematological malignancies. But the production process of CAR-T cells is complex and costly. Therefore, the development of in vivo in situ CAR-T therapy mediated by mRNA LNP is crucial.
A research and development team led by Drew Weissman and Michael J. Mitchell from the University of Pennsylvania has innovatively developed a strategy for antibody modified LNP (Ab LNP), achieving conceptual validation of in situ CAR-T therapy by targeting T cells with CAR mRNA.
Through customized CAR mRNA, such as tumor killing molecule CD19 and heart failure marker fibroblast activation protein (FAP), the in situ CAR-T platform is expected to be applied in the treatment of various diseases.
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



