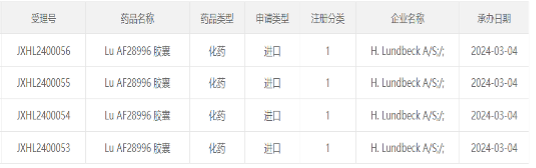
Lundbeck Pharmaceuticals’ “Anti-Parkinson’s Category 1 Chemical Drug” Submits Clinical Application in China
March 7, 2024
Source: drugdu
 585
585
March 4), according to the CDE official website, Lundbeck’s clinical trial application for Class 1 chemical drug Lu AF28996 capsules has been accepted. Currently, the drug is undergoing Phase I clinical trials overseas.
 Parkinson's disease is a neurological dysfunction disease with complex symptoms and difficult early diagnosis. It is common in middle-aged and elderly people. At present, the main treatment for Parkinson's disease is drug therapy, with the purpose of reducing symptoms, delaying the progression, and improving the patient's quality of life.
Parkinson's disease is a neurological dysfunction disease with complex symptoms and difficult early diagnosis. It is common in middle-aged and elderly people. At present, the main treatment for Parkinson's disease is drug therapy, with the purpose of reducing symptoms, delaying the progression, and improving the patient's quality of life.
Lu AF28996 is a dopamine D1/D2 receptor agonist developed by Lundbeck Pharmaceuticals. It is a relatively new anti-Parkinson therapy and is currently undergoing Phase I clinical trials overseas. Among them, a study to evaluate the safety, tolerability, etc. of Lu AF28996 in Parkinson's patients is expected to be completed in 2025.
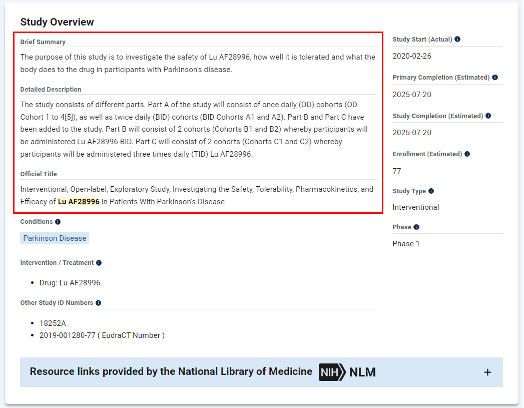 This time, the approval of Lu AF28996’s clinical trial application in China is expected to bring a new option to Parkinson’s patients.
This time, the approval of Lu AF28996’s clinical trial application in China is expected to bring a new option to Parkinson’s patients.
Lundbeck Pharmaceuticals is a century-old company located in Denmark. It has been deeply involved in brain diseases, especially mental diseases for decades. It has dozens of varieties on the market, including many blockbuster products. At the same time, Lundbeck is still focused on developing follow-up drugs in the field of psychiatric-nervous systems, and its clinical pipeline has full potential, covering treatments for depression, Alzheimer's disease, Parkinson's disease, and migraine. In addition, in 2023, Mr. Huo Yansi, general manager of Lundbeck China, said in an exclusive interview with the Embassy of the Kingdom of Denmark in China that China is currently Lundbeck's second largest market in the world.
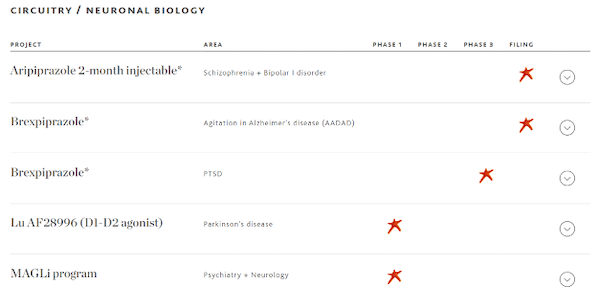
Brexpiprazole
Brexpiprazole, jointly developed by Lundbeck Pharmaceuticals and Otsuka Pharmaceuticals, is a second-generation (atypical) oral antipsychotic drug that has been approved as an add-on therapy for major depression and as a treatment for schizophrenia. In May 2023, Brexpiprazole was approved by the FDA for the treatment of agitation indications related to Alzheimer's disease. The drug became the first Alzheimer's disease agitation drug approved by the FDA.
Lu AG09222
Lu AG09222 is a monoclonal antibody targeting PACAP. At the 21st International Headache Conference, Lundbeck Pharmaceuticals presented the research results of the Phase IIa proof-of-concept trial of Lu AG09222 in migraine prevention. The research data supports Lu AGO9222 as a potential migraine prevention treatment. It is reported that Lundbeck Pharmaceuticals will conduct further trials of LuAG09222 based on this positive result to expand its route of administration opportunities and dose-response relationship.
In addition, Lundbeck Pharmaceuticals also has a number of potential products that are still in early clinical stages, including Lu AF28996, a new anti-Parkinson drug whose clinical trial application has been accepted.
It is understood that based on my country's huge population base, there are still a large number of unmet clinical needs in the field of brain disease treatment. In the future, as new treatments are continuously introduced, the treatment situation in related disease fields in my country is expected to be greatly improved.
https://news.yaozh.com/archive/42115.html
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



