Portable Rapid PCR Diagnostic to Detect Gonorrhea and Antibiotic Susceptibility
February 16, 2024
Source: drugdu
 418
418
Gonorrhea, ranked as the second most reported bacterial sexually transmitted infection (STI), affected approximately 82 million people globally in 2020. The infection can lead to severe health complications, including pelvic inflammatory disease, chronic pelvic pain, and infertility. Untreated gonorrhea may progress to the bloodstream, posing a life-threatening risk and increasing the likelihood of HIV infection. Many cases go unreported due to asymptomatic patients, implying that the actual burden of the disease may be significantly higher. Now, a rapid test aims to identify gonorrhea and also determine its antibiotic susceptibility.
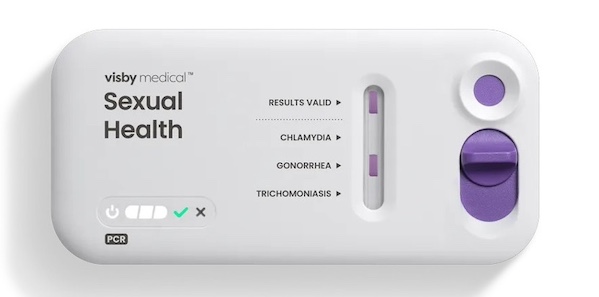
Visby Medical (San Jose, CA, USA) has been awarded up to USD 1.8 million by CARB-X (Boston, MA, USA) for the development of a portable rapid polymerase chain reaction (PCR) diagnostic to identify the presence of Neisseria gonorrhoeae (NG), the pathogen responsible for gonorrhea, and ascertain its susceptibility to ciprofloxacin. Ciprofloxacin was once a primary oral antibiotic for treating NG but has become less effective due to resistance. A rapid result indicating when ciprofloxacin may be effective would allow physicians to treat gonorrhea with greater confidence, reserving ceftriaxone for cases resistant to NG. Visby is renowned for its rapid and precise PCR testing capabilities for STIs, as well as COVID-19 and the flu. The funding from CARB-X will assist Visby in advancing to the next phase of development to address the pressing challenges in today's healthcare system.
Besides the development of a rapid test for NG and its susceptibility to ciprofloxacin, the funding will help Visby develop a test for NG as well as Chlamydia trachomatis (CT) and Trichomonas vaginalis (TV) in men based on urine samples. Visby currently offers a second-generation Sexual Health Test for the three most prevalent STIs in women, which is 510(k) cleared and has received a CLIA waiver from the U.S. Food and Drug Administration. Further CARB-X funding will be allocated in separate phases, contingent upon achieving specific project milestones, covering feasibility testing and development for antimicrobial resistance and male STI testing applications.
“The sexually transmitted infections epidemic continues to increase. That is why healthcare providers in ERs, urgent care clinics, community health centers and physicians’ offices need accurate and rapid diagnostic tests to enable same-visit, data-driven treatment based on a test result that identifies the pathogen and its antibiotic susceptibility," said Gary Schoolnik, MD, Chief Medical Officer of Visby Medical. “The CARB-X award will allow Visby Medical to enhance the only instrument-free PCR platform by adding the capacity to detect ciprofloxacin-resistant gonorrhea immediately and at the point-of-care. This has the potential to redefine best practice for patient care, infection control, and antibiotic stewardship.”“Our goal is to deliver diagnostics that are effective at all levels of the healthcare system,” added Erin Duffy, PhD, R&D Chief of CARB-X. “Given the portability of the envisioned Visby Medical PCR platform, which fits in the palm of your hand, we see this as rapidly and highly deployable in low-resourced and hard-to-reach settings. Additionally, for regions where ciprofloxacin remains a viable treatment, the Visby Medical diagnostic gives confidence that the physician is making the correct treatment decision.”
Source:
https://www.labmedica.com/microbiology/articles/294800262/portable-rapid-pcr-diagnostic-to-detect-gonorrhea-and-antibiotic-susceptibility.html
Read more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



