FDA Approves Expanded Indication for LEO Pharma’s Adbry for Pediatric Patients With Atopic Dermatitis
December 25, 2023
Source: drugdu
 338
338
Pharmaceutical Executive Editorial Staff
Indication of Adbry (tralokinumab-ldrm) expanded to include patients 12 to 17 years of age with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or for whom those therapies are not advisable.
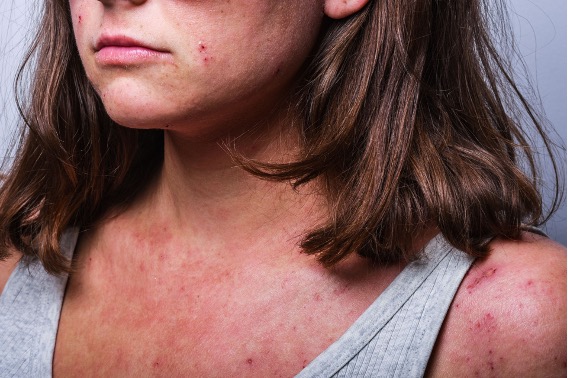 The FDA has approved an expanded indication for LEO Pharma’s Adbry (tralokinumab-ldrm) for patients 12 to 17 years of age with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or for whom those therapies are not advisable. Adbry is a human immunoglobulin G4 monoclonal antibody that specifically binds to human interleukin (IL)-13 and inhibits its interaction with the IL-13 receptor α1 and α2 subunits. Adbry is the first and only biologic FDA-approved for this indication.
The FDA has approved an expanded indication for LEO Pharma’s Adbry (tralokinumab-ldrm) for patients 12 to 17 years of age with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or for whom those therapies are not advisable. Adbry is a human immunoglobulin G4 monoclonal antibody that specifically binds to human interleukin (IL)-13 and inhibits its interaction with the IL-13 receptor α1 and α2 subunits. Adbry is the first and only biologic FDA-approved for this indication.
"This is an important milestone on our path towards making a fundamental difference for those who need it most. This critical patient group now has access to a much-needed additional treatment option to manage their atopic dermatitis," Brian Hilberdink, EVP and president, Region North America, LEO Pharma, said in a press release. “We are delighted to be able to offer Adbry, a highly targeted treatment, to both adult and pediatric patients in the US. This debilitating disease can have a particularly strong impact on pediatric patients, who often feel socially isolated because of their condition. We are incredibly proud of the progress we have made to date, and we will continue to work hard to address unmet needs in additional patient populations.”
In clinical trials, the drug produced significant improvements in itch, sleep interference, anxiety, depression, and overall quality of life among patients 12 to 17 years of age with moderate-to-severe AD. The expanded indication is for an initial loading dose of 300 mg followed by 150 mg dose every two weeks for this patient group.
The FDA based its approval for the expanded indication of Adbry on data from the Phase III ECZTRA 6 trial. Investigators evaluated the drug’s efficacy and safety in 289 patients aged 12-17 years with moderate-to-severe AD who were candidates for systemic therapy. Of those enrolled in the trial, 98 patients were administered an initial dose of Adbry 300 mg followed by 150 mg every other week up to week 16.
The study showed that 21% of patients administered Adbry achieved an Investigator's Global Assessment (IGA) score of 0 or 1 vs. 4% administered placebo. The trial also found that 29% of patients administered Adbry achieved at least a 75% improvement in Eczema Area and Severity Index score (EASI-75) vs. 6% administered placebo.
Further, 23% of patients administered Adbry achieved at least a four-point drop in Adolescent Worst Pruritis Numerical Rating Scale vs. 3% with placebo and a greater proportion of patients administered Adbry achieved at least a 90% improvement in EASI-90 compared to placebo.
“We know the symptoms associated with moderate-to-severe atopic dermatitis can have an impact on pediatric patients, which is why it’s so important to have treatment options with demonstrated efficacy in itch reduction and skin clearance,” said international coordinating investigator for ECZTRA 6, Amy Paller, MD, chair, Department of Dermatology, Feinberg School of Medicine, Northwestern University in Chicago, in a press release. “Clinical trial results that provide this evidence are invaluable to clinicians evaluating the safety and efficacy of treatment options for their pediatric patients.”
In terms of safety, the drug’s profile was comparable to what has been reported in clinical trials by adults with atopic dermatitis. The most common adverse events reported in adults were upper respiratory tract infections, conjunctivitis, injection site reactions, and eosinophilia.
“The approval of Adbry for pediatric patients living with moderate-to-severe atopic dermatitis expands the therapeutic options for those living with this disease who historically have had a limited selection to choose from,” said Julie Block, president and CEO of the National Eczema Association, said in a press release. “Such advances in the atopic dermatitis treatment landscape provide much-needed hope for pediatric patients seeking a long-term treatment option that could work for them.”
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



