Simcere and Connect sign licensing agreement for anti IL-4Rα AD drug
November 26, 2023
Source: drugdu
 399
399
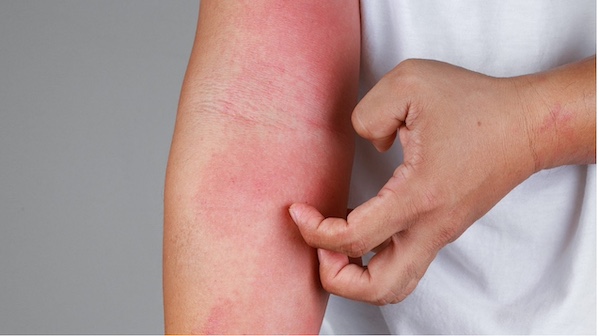
Simcere has entered a licensing agreement with Connect Biopharma for the development of a monoclonal antibody (MAb) candidate rademikibart (CBP-201) against allergic inflammation in autoimmune conditions such as atopic dermatitis (AD) and asthma.
Under the deal, the Nanjing, China-headquartered company will have a licence for the development and commercialisation of rademikibart in mainland China, Macau, Taiwan, and Hong Kong. Connect retains its rights to the MAb outside Greater China.
Rademikibart is a humanIgG4 MAb that binds to IL-4Rα, which can be administered as a subcutaneous or intravenous (IV) injection.
Sanofi and Regeneron Pharmaceuticals’ Dupixent (dupilumab) has dominated the AD drug market since it first gained a US Food and Drug Administration (FDA) approval in March 2017. Dupixent had generated $6.4bn in US sales up until 30 September, according to Regeneron’s Q3 2023 report. It is forecast to make over $11bn in global sales for all indications by the end of the year, according to GlobalData.
In November 2021, Connect announced topline positive results from the Phase IIb clinical trial (NCT04444752) of rademikibart in adult patients with AD. The global trial enrolled 226 from the US, China, Australia, and New Zealand. Patients were randomised to one of three treatment groups or the placebo group. The trial met its primary endpoint of percent reduction in eczema area and severity index (EASI) score from baseline to week 16, with a 73.7% reduction in the treatment group compared to 36.6% in the placebo group.
Connect is also investigating rademikibart in adult asthma patients. The ongoing Phase II study (NCT04773678) enrolled 306 patients with moderate to severe persistent asthma and is expected to conclude at the end of this year. The primary endpoint of the study is absolute change in prebronchodilator (trough) forced expiratory volume in the first second of expiration (FEV1).
In the announcement accompanying the agreement, Simcere chairman and CEO Jinsheng Ren said: “Rademikibart has demonstrated highly differentiated and best-in-class potential in Chinese AD patients. The addition of rademikibart expands our immune diseases portfolio, where we can leverage our knowledge and expertise to advance its program and bring it to market.”
https://www.pharmaceutical-technology.com/news/simcere-and-connect-sign-licensing-agreement-for-anti-il-4r%ce%b1-ad-drug/?cf-view
By editorRead more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



