CorMedix Wins FDA Approval for Antimicrobial Drug Combo After Two Rejections
November 18, 2023
Source: drugdu
 691
691
By Tristan Manalac
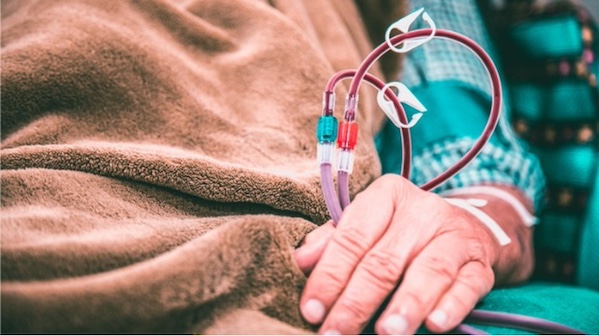
The FDA on Wednesday approved CorMedix’s DefenCath (taurolidine and heparin) to reduce catheter-related blood stream infections in adults with kidney failure who are on chronic hemodialysis through a central venous catheter.
DefenCath is the first FDA-approved antimicrobial catheter lock solution in the U.S., according to CorMedix’s announcement. CorMedix expects DefenCath to be available in the inpatient setting in the first quarter of 2024. The company’s stock was trading 35% higher Wednesday morning in response to the approval, according to Seeking Alpha.
CorMedix CEO Joseph Todisco in a statement said that DefenCath’s approval was a “major advancement” for preventing life-threatening infections and provides doctors “an option to reduce the risk of infections in a patient population already vulnerable due to underlying kidney failure.”
DefenCath is a combination of the amino acid derivative taurolidine and the anticoagulant heparin. Taurolidine has demonstrated strong antimicrobial activity against gram-positive and gram-negative bacteria, including those that have already evolved some forms of antibiotic resistance. Taurolidine is also potent against Aspergillus infections and other clinically important fungi.
However, it has been a challenging regulatory path for CorMedix. The FDA rejected DefenCath twice.
CorMedix received the first Complete Response Letter (CRL) in March 2021, in which the regulator flagged “concerns” at a third-party manufacturer. The company was hit with the second CRL in August 2022, which was likewise connected with problems at a contract manufacturer, as well as issues with its heparin supplier.
In March 2023, CorMedix announced that both the third-party service provider and the supplier had implemented corrective measures in line with the FDA’s guidance. The regulator accepted CorMedix’s resubmission in June 2023 and classified it as complete.
CorMedix supported DefenCath’s application with data from the Phase III LOCK-IT-100 trial, a randomized, double-blinded and active-control study, which showed that DefenCath lowered the incidence of catheter-related bloodstream infections by 71% versus heparin alone. The strong evidence of efficacy led an Independent Data Safety and Monitoring Board to recommend the study’s early end.
DefenCath is also approved under the FDA’s Limited Population Pathway for Antibacterial and Antifungal Drugs. According to the regulator’s website, this pathway allows the approval of drugs “based on a benefit-risk assessment that more flexibly considered the severity, rarity, or prevalence of the infection” that the agent is designed to address, along with the lack of viable alternative treatments the specific patient population targeted.
The label for DefenCath does not bear a boxed warning but carries precautions against heparin-induced thrombocytopenia and hypersensitivity reactions. DefenCath is contraindicated for those with known hypersensitivities to its components and to pork products.
Read more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



