Janssen Sends Data for Phase III Children’s Pulmonary Tuberculosis Treatment to FDA and EMA
November 10, 2023
Source: drugdu
 448
448
Don Tracy, Associate Editor
Company aims to receive approval on a Type II Variation application for Sirturo for patients with pulmonary tuberculosis.
The Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen) has announced the submission of a Type II Variation application to the European Medicines Agency (EMA) for bedaquiline (Sirturo), indicated as part of a combination therapy for adults and pediatric patients over the age of five with pulmonary tuberculosis (TB) due to multi-drug resistant Mycobacterium tuberculosis. Back in August, a supplemental New Drug Application (sNDA) was also submitted to the FDA for the medication.
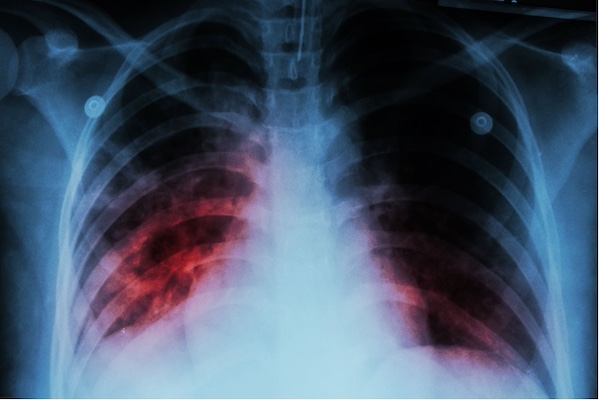
According to data from the CDC, there was a report of approximately 8,300 cases of TB in 2022, up from 7,874 cases reported in 2021. The center also reports that TB cases in the United States are beginning to return to pre-pandemic levels, following a substantial decline in 2020, likely due to factors associated with the COVID-19 pandemic, including missed or delayed diagnoses.2 A total of 1.3 million people worldwide died of TB in 2022 (including 167,000 patients with HIV), and the disease is considered the second leading infectious killer after COVID-19—above HIV and AIDS.3
Sirturo is a diarylquinoline antimycobacterial medication that indicated as part of combination treatment in adults with pulmonary MDR-TB. The FDA has noted that Sirturo use should be reserved for cases in which an effective treatment regimen cannot otherwise be provided.
The drug includes Boxed Warnings for an increased risk of death and occurrence of QT prolongation. The Warnings and Precautions section also includes the risk of hepatic-related adverse events (AEs), drug interactions, use in HIV-TB coinfected patients and treatment failure. The most common AEs reported with Sirturo are nausea, arthralgia and headache, as well as hemoptysis and chest pain.
Janssen’s application includes data from its Phase 3 Stream Stage 2 study (NCT02409290), which is a Post-Marketing Requirement in the United States, a Specific Obligation in the EU, and a Post-Marketing Commitment in several other countries. According to the company, its study compared an all-oral, nine-month Sirturo-containing regimen to the control regimen of a nine-month injectable-based regimen for the treatment of TB (MDR-TB).
Sirturo was first granted accelerated approval by the FDA in December 2012 and conditional approval by the EMA in March 2014 following positive Phase 2 study data. If the application is approved by both agencies, they will support traditional approval in the United States and full approval in the EU.
By editorRead more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



