Acelyrin’s Lead Asset Izokibep Fails in Late-Stage Hidradenitis Suppurativa Trial
September 14, 2023
Source: drugdu
 368
368
By Tristan Manalac
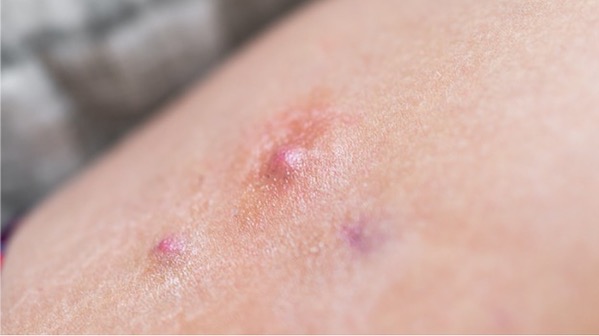 Pictured: Close-up of a skin lesion in a patient with hidradenitis suppurativa/iStock, krblokhin
Pictured: Close-up of a skin lesion in a patient with hidradenitis suppurativa/iStock, krblokhin
Top-line data from a Phase IIb/III trial showed that Acelyrin’s investigational IL-17A inhibitor izokibep fell short of its primary endpoint in patients with hidradenitis suppurativa, a chronic inflammatory skin condition, the company announced Monday.
In patients who were being treated with 160-mg izokibep once-weekly, 39% demonstrated at least a 75% decrease in the hidradenitis suppurativa clinical response (HiSCR75) score at 16 weeks, while 29% of placebo comparators achieved a similar level of clinical response. This did not result in a significant treatment effect, with a p-value of 0.3278, according to the company’s announcement.
Even the twice-weekly izokibep schedule was unable to significantly distinguish itself from placebo, with only 34% of patients achieving HiSCR75 at 16 weeks. These findings were calculated using a non-responder imputation (NRI) method.
Acelyrin’s shares tanked 64% in after-hours trading in reaction to the news.
Monday’s data came from Part B of a double-blinded and placebo-controlled Phase IIb/III study that enrolled 175 patients with moderate-to-severe hidradenitis suppurativa. Izokibep was given at 160-mg doses either once- or twice-weekly.
The study’s primary endpoint was HiSCR75 as calculated by NRI, which according to Acelyrin was negatively affected in Part B by high rates of discontinuation as early as four weeks into the trial. The company also pointed to an unexpected increase in placebo response, leading to izokibep’s non-significant treatment effect.
To account for the discontinuations, Acelyrin also employed the last observation carried forward (LOCF) sensitivity method, which found that the once-weekly schedule was significantly better than placebo at inducing HiSCR75 at week 16.
In addition, a modified NRI analysis—likewise accounting for drop-outs—found that izokibep elicited higher rates of HiSCR75 than placebo with strong statistical significance.
The California biopharma also detected stronger signals of efficacy in terms of HiSCR100—representing a 100% decrease in scores from baseline—though izokibep still fell short of statistical significance in both NRI and LOCF analyses.
When it came to safety, izokibep’s profile was consistent with what had been previously established. Thirty-one patients dropped out of the study, the majority of which were not attributable to side effects.
Despite failing its primary endpoint, Acelyrin CEO Shao-Lee Lin remained encouraged by izokibep’s “consistent early and high orders of response,” she said in a statement. “The consistent and early achievement of HiSCR100, along with our prior izokibep experience in Psoriatic Arthritis, continues to demonstrate the potential of izokibep for resolution of disease, especially in difficult to treat tissues.”
Acelyrin has an ongoing Phase III izokibep trial in hidradenitis suppurativa, which it initiated in June 2023. The company also completed its IPO in May 2023, raising a total of $540 million.
Read more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



