Abiomed heart pump recall labeled Class I by FDA, no deaths reported
June 7, 2023
Source: drugdu
 295
295
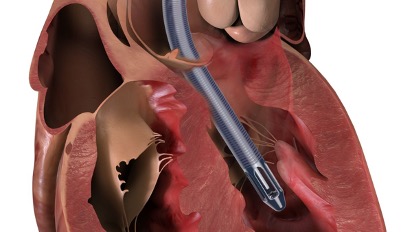
The FDA said Monday that Abiomed, the heart device maker bought by Johnson & Johnson last year for $16.6 billion, is recalling some sets of the Impella 5.5 heart pump with the SmartAssist function after receiving complaints that purge fluid has leaked from the purge sidearm of the pump.
The FDA has identified the action as a Class I recall, the most serious kind, meaning that continued use may cause serious injuries or death, unless corrective measures are taken.
The Impella 5.5 with SmartAssist System is used for up to 14 days to support the pumping chambers of the heart when there is ongoing cardiogenic shock that occurs less than 48 hours after a severe heart attack, open-heart surgery, or when the heart is not functioning well due to a condition called cardiomyopathy, the FDA said in a statement.
The FDA warned that if a purge leak occurs, “the system will experience low purge pressures,” prompting alarms and requiring evaluation. In patients who are in critical condition, the agency said failure of the pump’s support can lead to “further deterioration and worsening of their already critical condition and may even lead to serious injury or death.”
Abiomed initiated the recall in April. The company has reported 179 complaints, three injuries and no deaths related to the recall, the FDA said.
“Abiomed has notified customers and the FDA about this voluntary recall,” Jenny Leary, the company’s associate director of U.S. communications, wrote in an email, adding that the latest versions of the Impella 5.5 with SmartAssist Sets are not part of the recall.
Updates with comment from Abiomed.
Reference:https://www.medtechdive.com/news/JNJ-Abiomed-Impella-recall/652103/
By editorRead more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



