Formus Labs’ Hip Surgery Planning Software Expands with FDA Clearance
May 7, 2023
Source: drugdu
 471
471
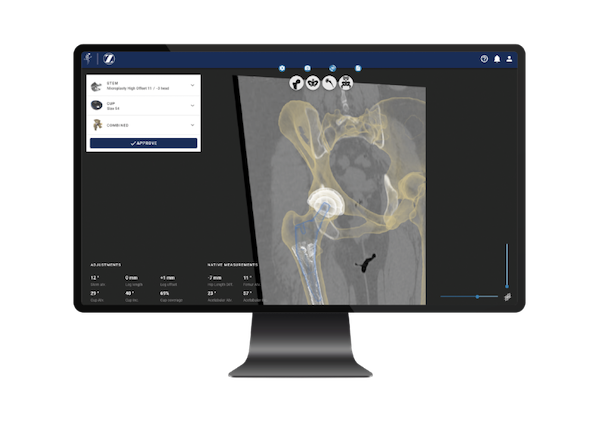
In order to carry out a seamless joint replacement, surgeons must create a customized preoperative plan for each patient. However, surgeons having to perform multiple operations per day are often too busy to dedicate adequate time to this step.
Formus Labs, a New Zealand-based medical technology startup, is on a mission to make the preoperative planning process much quicker. On Wednesday, the company received 510(k) clearance from the FDA for Formus Hip, its fully automated 3D software for hip replacement preoperative planning.
The company announced its plans to expand into the U.S. last year. Wednesday’s FDA clearance represents a major milestone, as Formus can now begin to sell its product to surgeons and healthcare providers across the country.
Founded in 2017, Formus seeks to solve the problem of patient dissatisfaction and unsustainable costs in joint replacement surgery, said CEO Ju Zhang in a recent interview.
“Our approach to solving this problem is by predicting how to perform the best joint replacement surgery for each individual patient based on their form and function, in an automated way that is scalable to every patient,” he said.
To make the joint replacement planning process faster, Formus Hip combines AI and computational biomechanics to calculate a patient’s implant fit and produce interactive 3D surgical plans.
The software first extracts a patient’s unique 3D anatomy from their CT scan, then it simulates the fit of a catalog of implants to that patient’s anatomy. Based on the fit and the surgeon’s preferences, Formus Hip recommends the best implants for the patient. The surgeon is then able to review the patient’s anatomy and recommendations for implants and make changes as needed, Zhang explained.
The surgeon also receives a report with the key information needed to execute the surgery. These reports include the patient’s recommended implant types and sizes, their positions and orientations, intraoperative measurements useful for the delivery of those implants, the patient’s predicted postoperative measurements, and images to guide the placement of the implants.
“Unlike other planners and planning services that take days or weeks to create a plan, Formus Hip goes from scan to plan automatically in under an hour, freeing the surgeon and their team to focus on their patients. This automation also means that highly accurate 3D pre-operative plans are now scalable to every surgeon and every patient,” Zhang said.
The medical software company has already planned thousands of surgeries in New Zealand, he pointed out. The startup also struck a partnership last year with Zimmer Biomet, which makes hip and knee implants, to commercialize Formus Hip first in Australia and New Zealand and later in global markets.
Zhang said the company is excited to bring Formus Hip to the U.S., where it hopes to start piloting usage at healthcare sites in the next few months.
But the startup is far from the only company focused on preoperative surgical planning, be it in joint replacement or another area. For example, virtual reality companies like Osso VR and FundamentalVR train surgeons to help them hone their skills. And Formus competes with other 3D planning softwares like MediCAD, PeekMed and ZedHip.
But Zhang points to AI as the point of differentiating from these companies.
“What sets us apart is our ability to deliver a plan without anyone doing the planning — not the surgeon, not the rep and not some engineer on the other side of the world. The time and cost savings are on the order of days and thousands of dollars, respectively, per surgery,” Zhang declared.
Formus also believes that “less is more” when it comes to medical software, and the company takes pride in its simple, intuitive user experience, he added. Zhang called the startup’s user interface “a stark departure from the cluttered, dated design of many of [its] competitors.”
Reference:https://medcitynews.com/2023/05/fda-hip-surgery-joint-replacement-new-zealand/
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



