Scientists develop fastest calcium indicators yet for neural imaging
April 6, 2023
Source: drugdu
 441
441
by Howard Hughes Medical Institute
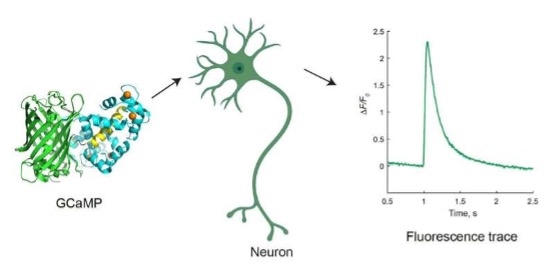
Overview of GCaMP calcium indictors for neuronal imaging. When they sense calcium, GCaMP indicators cause neurons to produce green fluorescent light, allowing scientists to see which neurons and synapses are activated in living animals as they perform a task. Credit: Yan Zhang
New ultra-fast sensors developed at Janelia can detect calcium ions nearly as fast as they are released from neurons, allowing scientists to tease out the individual, milliseconds-long signals passing between brain cells.
Genetically encoded calcium indicators dubbed GCaMPs are used to track the activity of large populations of neurons in living animals by revealing the calcium ions that are released as signals pass between neurons. When they sense calcium, GCaMP indicators cause the neurons to produce green fluorescent light, allowing scientists to see which neurons and synapses are activated in living animals as they perform a task.
GCaMP indicators have become brighter and more sensitive since they were first developed more than 20 years ago. But even state-of-the-art calcium sensors haven't been fast enough to tease out the individual signals of specific neurons being activated. That left scientists able to track only groups of neurons and regions of the brain.
Now, Janelia researchers have created several new versions of the GCaMP sensors that can capture neuronal signals almost as fast as they happen. The new jGCaMP8 sensors, which are nearly 10 times faster than previous GCaMPs, are detailed in a study published March 15 in Nature.
The ultra-fast speed of the jGCaMP8 indicators, along with their high sensitivity, means scientists can now disentangle individual signals coming from specific neurons, enabling researchers to better understand the brain signals engaged when a fly flaps its wings, a mouse twitches its whiskers, or a fish waggles its fins.
Janelia scientists have developed new ultra-fast genetically encoded calcium indicators that can detect calcium ions nearly as fast as they are released from neurons. In this movie, ultra-fast jGCaMP8 indicators were used to track the response in L2 neurons that are part of the “off” pathway that detects changes in light intensity in the fruit fly visual system.The top image shows the changes in pixel intensity from L2 neurons as light is dimmed and brightened. When the light is turned off, the neurons depolarize, calcium concentrations increase and GCaMP fluorescence increases. When the light is turned on, the neurons hyperpolarize, calcium concentrations decrease and GCaMP fluorescence decreases. The bottom graph shows the mean change in intensity from the outlined region of interest. Credit: Daniel Bushey
"This is a new regime for functional imaging, to be at these timescales," says Loren Looger, a former Janelia Group Leader who is now an HHMI Investigator at the University of California, San Diego. "Now you can start to ask the questions that are really at the nuts and bolts of how neural computations take place."
A primer on GCaMPs
Since 2006, Janelia scientists have been working to optimize GCaMP sensors in an effort led by Looger, the GENIE Project Team, and close collaborators working with different model organisms, including former Group Leader Karel Svoboda, Senior Group Leader and Head of Mechanistic Cognitive Neuroscience Vivek Jayaraman, Group Leader Glenn Turner, and Senior Group Leader Misha Ahrens. The project has produced brighter and more sensitive indicators that are considered one of the best ways to image large populations of neurons simultaneously in live animals, according to Looger.
But though the GCaMP indicators improved in many ways over the years, they had not gotten much faster. While a signal passes between neurons at a speed of about 2 milliseconds, the GCaMP indicators would produce a signal starting after about 50 milliseconds and lasting about 600 milliseconds. Scientists were able to see that a group of neurons had been activated, but they couldn't tease out the individual spikes making up the signal.
Janelia researchers and other teams tried to make faster GCaMP indicators, but the increased speed came at the expense of sensitivity and brightness. Then, six years ago, Looger and Janelia Senior Scientist Yan Zhang decided to try again.
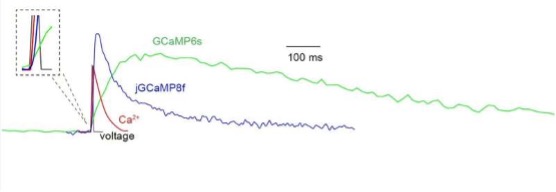
Schematic representation of changes during action potential. This schematic shows waveforms of membrane potential (black), calcium ions (red), GCaMP6s fluorescence (green) and jGCaMP8f fluorescence (blue) in a cortical pyramidal neuron following a single action potential. jGCaMP8f follows the calcium ions much more closely than GCaMP6s. Credit: Yan Zhang
Making a faster GCaMP
The team started by looking at the specialized structure of the GCaMP indicators. The part binding to the calcium contains the protein calmodulin and a peptide, RS20. Once bound to calcium, calmodulin and RS20 interact to produce a change in the protein shape that causes the connected Green Fluorescent Protein, or GFP, to fluoresce more brightly.
This interaction between calmodulin and the peptide controls the speed of the GCaMP sensor. By changing RS20 to another peptide, the team thought they might be able to speed up the indicator. They tried 30 different peptides and found one that sped up the GCaMP signal without losing too much sensitivity.
Next, the team sought to improve the sensitivity by swapping out other parts of the protein complex, called the "linkers" and "interface." They worked with the GENIE Project Team to test 1,000 different mutations in cultured neurons. Finally, after four years, they had an ultra-fast indicator that was more sensitive than previous GCaMPs. They released the initial jGCaMP8 sensors, with three variants for different applications, in 2020.
"The previous GCaMPs were too slow to capture the entire neuron signal," says Zhang, a lead author of the new paper. "jGCaMP8 has a much faster rise time in its fluorescence signal—for some applications it has even a 2-millisecond half-rise time." Due to the improved speed, she notes, the sensor follows the change in calcium concentration more precisely. "Contrary to previous attempts of speeding up GCaMPs, which compromised sensitivity, the new jGCaMP8 can produce even higher sensitivity, as it captures much more of the calcium signal during neuronal activities."
The increased sensitivity and speed of the jGCaMP8 sensors will enable researchers to perform experiments they couldn't do before, says Márton Rózsa, a neuroscientist at the Allen Institute and a lead author of the new paper. Previous sensors only allowed researchers to see bursts of activity when recording populations of neurons, but jGCaMP8 enables them to precisely detect single action potentials of individual neurons at larger scales.
"We want to understand how the brain processes information, and if we are not recording all of the information that is present in the brain, then we will likely construct a false model," Rózsa says. "The more information we get, the better models we can build, and this sensor is really pushing us in that direction."
Looger believes the development of jGCaMP8 could only have happened at a research institution like Janelia, where labs have the support of Shared Resources and Project Teams—as well as the subject-matter expertise of the labs working on specific model organisms—to take on major projects that require time, resources, and expertise from many different people.
"If one lab tried to do it themselves, it would have been impossible, but even if it was possible, it would have taken five times as long and cost five times as much," he says. "It really does take someplace like Janelia."
By editorRead more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



